Page 10 - hypertension_newsletter8
P. 10
REFLECTIONS
Hypertension
Hypertension Global Newsletter #8 2025
The study was conducted using a parallel design, a randomised clinical trial at 145 clinical sites located in different regions across
China. Patients with T2D, 50 years of age and older, an elevated SBP, and deemed to have an increased risk of cardiovascular Hypertension
disease were enrolled and randomly assigned to receive either intensive or standard blood pressure treatment for up to five years.
Elevated SBP was defined as 130 to 180 mmHg in patients taking antihypertensive medications or at least 140 mmHg in patients not
taking medications. The primary outcome was a composite of the first occurrence of non-fatal stroke, non-fatal myocardial infarction,
treatment or hospitalisation for HF, or death from cardiovascular causes.
A total of 12,821 patients were enrolled; 6414
were assigned to the intensive treatment
group, and 6407 were assigned to the standard
treatment group. The mean age of the patients
was 63.8 years, 54.7% of the patients were
male, and 22.5% had a history of clinical CVD
at baseline. A total of 1180 patients either
discontinued the trial, were lost to follow-up, or
withdrew consent.
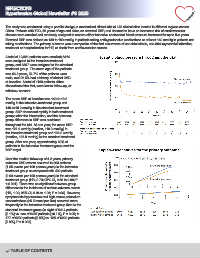
The mean SBP at baseline was 140.0±10.2
mmHg in the intensive-treatment group and
140.4±10.2 mmHg in the standard-treatment
group. SBP decreased rapidly in both treatment
groups after the intervention, and the between-
group difference in SBP was sustained
throughout the trial. At one year, the mean SBP
was 121.6 mmHg (median, 118.3 mmHg) in
the intensive treatment group and 133.2 mmHg
(median, 135.0 mmHg) in the standard treatment
group. After one year, approximately 60% of
patients in the intensive treatment group met the
SBP target.
Over the median follow-up of 4.2 years, primary
outcome CVD events occurred in 393 patients
(1.65 events per 100 person-years) in the intensive
treatment group as compared with 492 patients
(2.09 events per 100 person-years) in the standard
treatment group (HR, 0.79; 95% CI, 0.69 to 0.90; P
< 0.001). There was no significant between-group
difference in the incidence of serious adverse events
(HR, 1.00; 95% CI, 0.94 to 1.06; P = 0.96). However,
symptomatic hypotension and high serum potassium
concentrations (>5.5 mmol per litre) occurred more
frequently in the intensive treatment group than in the
standard treatment group (in eight of 6414 patients
[0.1%] vs. one of 6407 patients [<0.1%], P = 0.05; in
177 of 6230 patients [2.8%] vs. 125 of 6220 patients
[2.0%], P = 0.003).
TABLE OF CONTENTS